Formula for Change in Internal Energy
ICP is a fast-growing trend. This energy is a measure of the forces that hold the nucleons together.

Calculating Internal Energy And Fluid Temperature Using The Nfee Youtube
This mass change must be released as various types of photon or other particle energy as above according to the relation E mc 2.
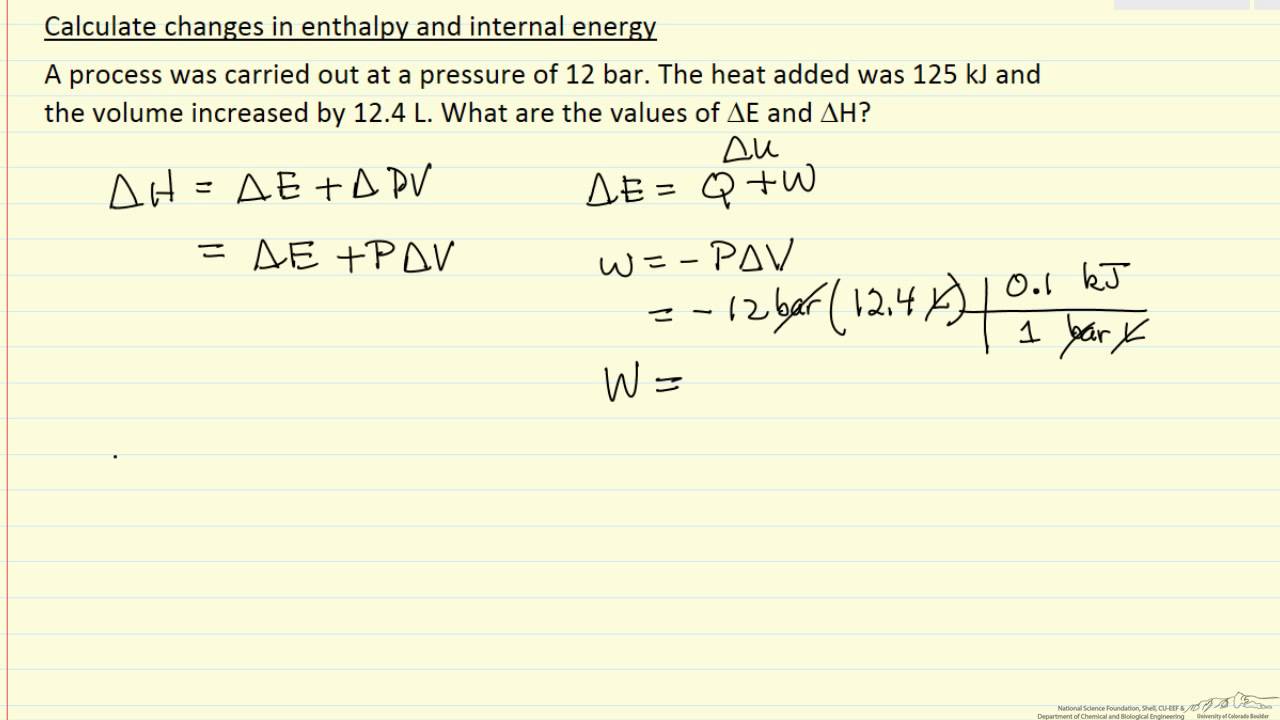
. In 2020 some 5900 companies reported carbon pricing data while research by the Carbon Disclosure Project CDP showed the number of companies using or planning to use ICP has. ΔU q W. Internal Energy of a Closed System.
Now from the above one can derive the dimensional formula of length left M0L1T0 right Finally the formula of strain is fracchange in dimensionoriginal value of dimension Solved Example on Strain Formula Q1 Heating results in the expansion of metals. Thus after the binding energy has been removed binding energy mass change c 2. A hot liquid enters through a copper pipe 1000 m long.
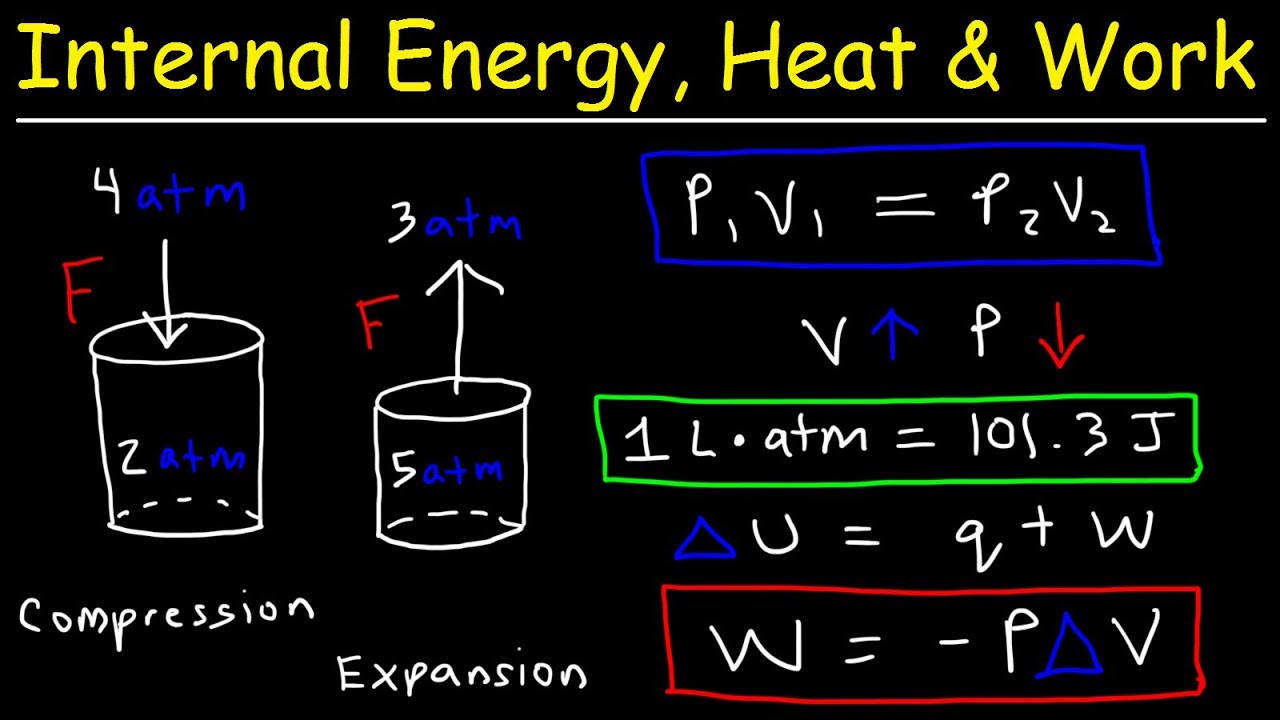
It represents energy that must be resupplied from the. For a closed system the internal energy is essentially defined by. - electrical work wQ I Q is charge in coulombs I.
ΔH ΔQ p ΔV. U is the change in internal energy of a system during a process. Using reaction scheme Determine change in enthalpy of the chemical reaction as follows.
Work done on the system raises internal energy of system w. As we know that the enthalpy change formula is given as. Internal Energy Work Heat and Enthalpy 15 More general formula for PV work P does not need to be constant f i V V ext w P dV ³ Sign Convention.
By putting the values of pressure internal energy and change in volume we can calculate change in enthalpy of the system as follows. The most widely used approaches by business are internal carbon pricing ICP and include hypothetical cost of carbon offsetting and internal carbon taxes or levies. 0 Work done by the system lowers the internal energy w 0 Other forms of work.
If the internal energy is expressed on an amount of substance basis then it could be referred to as molar internal energy and the unit would be the Jmol.
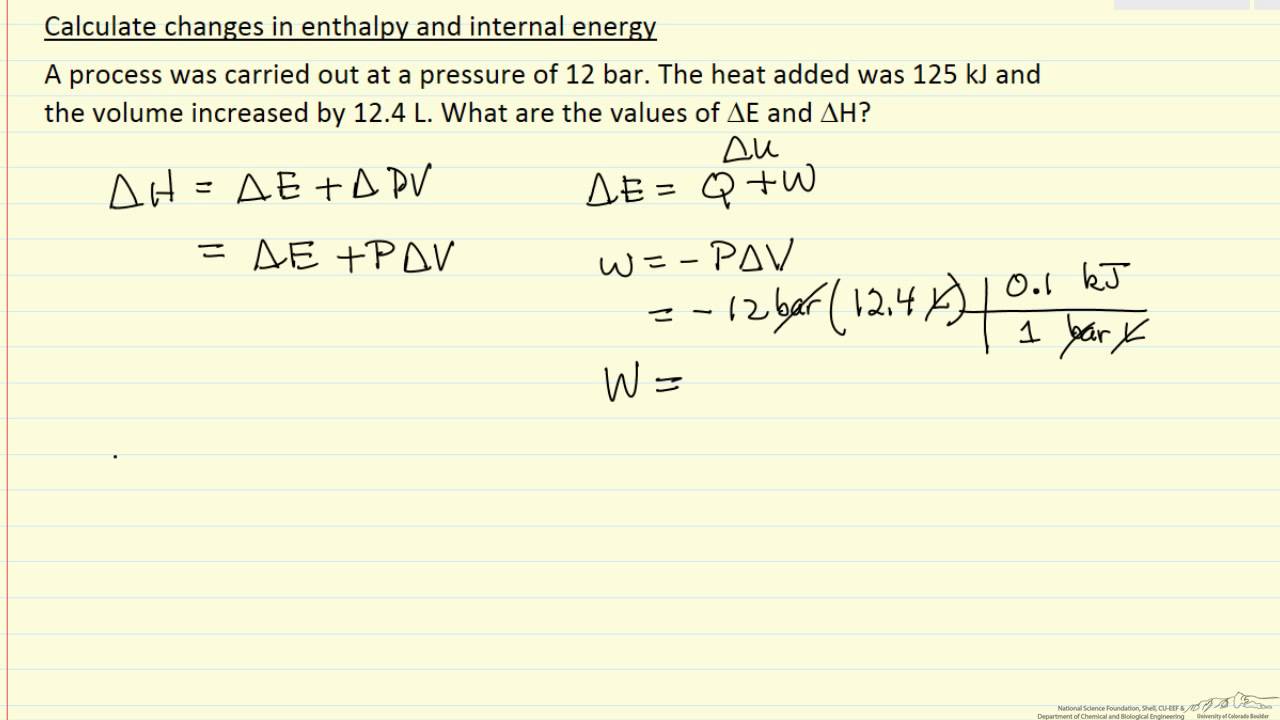
Changes In Enthalpy And Internal Energy Example Youtube
Internal Energy Heat And Work Thermodynamics Pressure Volume Chemistry Problems Youtube

Chapter 3a The First Law Closed Systems Energy Updated 1 17 11
Comments
Post a Comment